- Nov 6, 2009
- 1,511
- 73
- 91
The Wired article didn't seem to mention any alternatives to using wax to absorb the momentary extra heat during the sprint.
But are there, or could there be developed, other materials like crystalline structures that absorb heat until they melt? Why is wax the best choice here to absorb lots of sprinting heat until it melts?
The Wired article didn't seem to mention any alternatives to using wax to absorb the momentary extra heat during the sprint.
But are there, or could there be developed, other materials like crystalline structures that absorb heat until they melt? Why is wax the best choice here to absorb lots of sprinting heat until it melts?

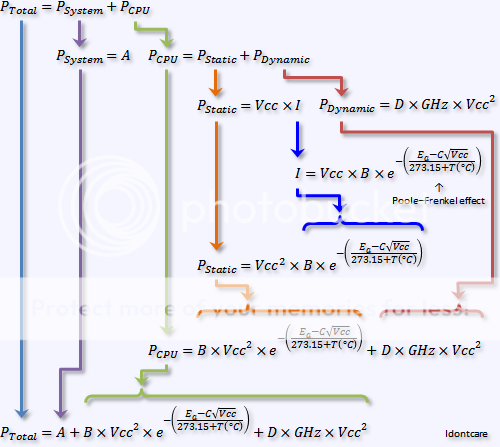
Yeah the point isn't to use a material which has a high heat capacity, but rather to use a material which has a high enthalpy of fusion.
High heat capacity just means the temperature keeps rising as you dump more heat into it, but the rate of increase in temperature will be low(er) because the heat capacity is higher.
Water is great in this regard.
But that doesn't keep your chip from over-heating and becoming a 90C furnace in your hand though.
To prevent the actual temperature from going up you need a material which is absorbing the heat while melting, because materials that melt do so at a fixed temperature.
Take ice for example, it doesn't matter how much heat or how fast you push the heat (within reason, no nuclear bombs obviously) into ice, the temperature will remain a fixed 0 Celsius until the ice has absorbed enough heat as to convert all the ice to liquid water, after which the temperature will then rise in proportion to its heat capacity.
Here is the heat absorbed vs. temperature for water:

A, C, and E are heat capacity - how much heat does it take to increase the temperature of ice, liquid water, and steam respectively?
B and D heats of fusion and vaporization respectively - how much heat does it take to melt ice and to boil water.
Notice how the temperature flatlines at those two phase transitions for the material despite all the heat being pumped into the system?
So the idea here is to have your sprinting microprocessor kept nice and cool while it is sprinting (lower leakage and more comfortable for a given form factor like being held by your hand) because you choose a material which has a low melting point and has a large enthalpy of fusion.
The nice thing about paraffin is that it takes a lot of energy to convert it from a solid to a liquid. It'll hit its melting temperature, and stay there until enough energy is accumulated to turn it into a liquid.I'm assuming because the wax is fairly cheap and it has a nice melting temp of 54C. It is a tad high imo for mobile devices, but it would seemingly do the trick. I'm by no means a materials scientist, but I can't think of anything else that is in the same price range with a good melting point. All I could find in the presentation linked was them referring to using a phase change material, and no specifics.
If I understand correctly, you need something that has a melting point as close to ambient as possible, while still being above it. Ice for example doesn't work because it is already liquid water at relevant temperatures. It also needs to have a high heat of fusion -- water has this, but both criteria need to be met. Silicon itself has a fantastically high heat of fusion at 1926 J/g, but because it doesn't melt until 1687K, it's not very useful for keeping temperatures around ambientExactly, which is why I can't help but think a crystalline structure (like ice) would be excellent. Your wikipedia link to enthalpy of fusion suggests that wax is good, but crytalline structures are better? Sorry I'm not proficient in this subject matter, but it's very interesting.
If I understand correctly, you need something that has a melting point as close to ambient as possible, while still being above it. Ice for example doesn't work because it is already liquid water at relevant temperatures. It also needs to have a high heat of fusion -- water has this, but both criteria need to be met. Silicon itself has a fantastically high heat of fusion at 1926 J/g, but because it doesn't melt until 1687K, it's not very useful for keeping temperatures around ambient
Ah yes... good ol' chemistry.
Two questions:
1) If all of the paraffin is melted (which would take a good amount of energy, no doubt), you're looking at 2.13 J/g-K of specific heat, roughly half the amount of water. What cools down the wax then? Would the heat dissipation of the wax fast enough to bring it back down to a solid state before the next sprinting cycle? Unless the whole idea is to never have all of the wax melt...
2) From what I understand, power (and therefore heat produced) scales exponentially with frequency. I understand that it can be better to "sprint" fast and park sooner than to "jog" and take longer to park, but can this philosophy really save energy if taken to the extreme as in the case of 100W smartphone processors? I figure that of the 100W, the last 30-40W or so only improves the clock rate of the chip by only 10-15% due to diminishing returns. Isn't this why Netburst was scrapped? It seems the whole point of this implementation is to be able to get snappier loading of web pages and whatnot but at the loss of power efficiency.


... Oooh, maybe they can infuse the wax with diamond dust or other particles to give the wax great thermal heat transfer properties like a heatsink, in addition to it's ability to guzzle down heat while melting?


